E2B(R3) - Stay compliant
We are a frontrunner in designing and implementing globally integrated E2B(R3) solutions for our clients
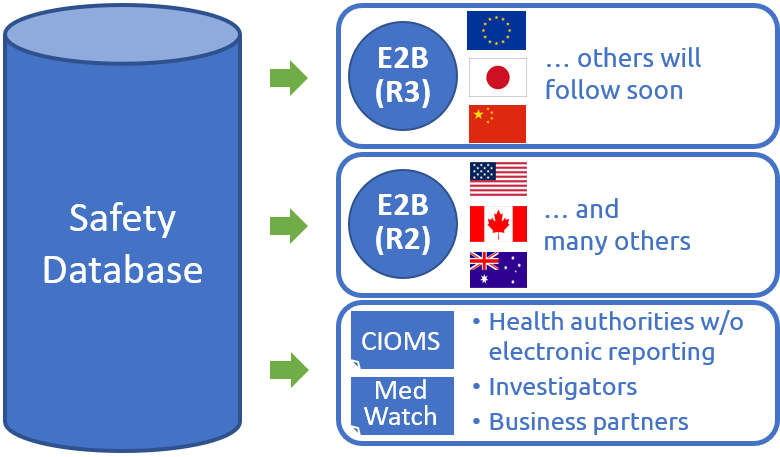
ICSR reporting in the new E2B(R3) standard becomes mandatory at many Health Authorities within the upcoming years. For many pharma companies, the implementation of the new reporting standard is a challenge due to it’s technical complexity and existence of different regional variations (e.g. Europe, Japan, China). This leads to significant complexity for implementation projects and definition of harmonized global data entry processes.
With one of our clients, we have successfully implemented one of the first truely integrated solution for E2B(R3) reporting from a single safety database to EMA (Europa) and PMDA (Japan) while staying compliant with remaining E2B(R2), CIOMS, MedWatch and other reporting standards.
With this experience, we can support you in designing a modern safety database in your environment which is able to cover all necessary reporting standards in parallel, such as E2B(R3), E2B(R2), CIOMS, MedWatch and others. We enable your PV organization to serve all required output formats from a single safety database solution.
We are also monitoring upcoming E2B(R3) requirements, especially in USA, South Korea, Brazil and others. For all upcoming requirements, we strive for globally integrated solutions to reduce risks of isolated local solutions in a global context.