Risk Management
A supply chain is only as strong as its weakest link
JSC provides proven strategies in the (bio)pharmaceutical industry to ensure reliable market supply of (key) products in tow with patient and business needs
Risks can arise due to e.g. natural disasters, epidemics / pandemics, civil unrest, regulatory issues, chemical accidents, infrastructure failures, quality and EH&S issues or financial problems.
In order to be effective, risk management must follow a holistic approach considering the entire end-to-end supply chain from prelaunch to withdrawal, and systematically analyze risks aligning all concerned parties.
Risk management processes should be established and managed according to ICH Q9 comprising risk assessment, risk control, monitoring and reporting as well as setting the risk management framework.
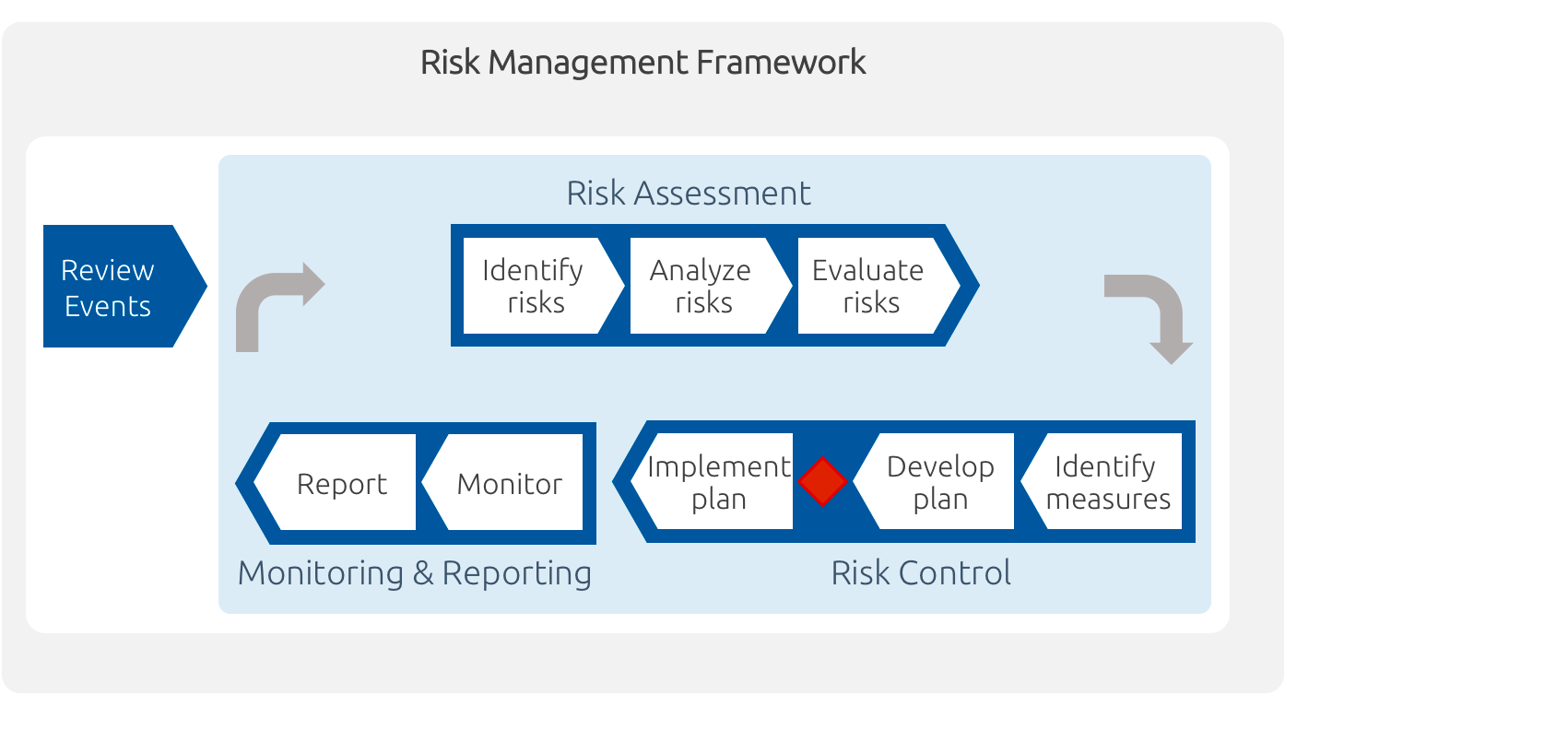
When JSC implements risk management, we always adhere to the following principles:
- Closely integrate risk management in the organization – it needs to be considered in decision making at all levels
- Focus on products with major impact on medical health and / or high commercial relevance
- Establish a continuous process: regularly iterate risk assessments and monitor implementation of risk reduction measures
- Prepare for mid-term interruptions – temporary issues are covered by day to day operation, force majeure risks / disasters are very rare
- Use released stock as a key element of contingency plans – especially regarding APIs, KSM and products in early lifecycle phase
- Implement redundant structures: register 2 suppliers, follow multi-site approach for standard, multi-plant at one site approach for non-standard processes

IT Systemunterstützung im Qualitätsrisikomanagement – Beispiel: “Supply Chain Integrity” (German)

API Contingency Concept – JSC worked with one of the top global pharma company to enhance its contingency planning for API supply


